Equiptrack products and services are based only on information supplied to Equiptrack. Equiptrack does not have the complete history of every unique piece of equipment. Use the Equiptrack search as one important tool, along with an equipment inspection and functional check, to make a better decision about purchasing your next piece of used medical equipment.
WHAT IS THIS?
A Recall is a firm’s removal or correction of a marketed product that the FDA considers to be in violation of the laws it administers and against which the agency would initiate legal action, e.g., seizure. Recall does not include a market withdrawal or a stock recovery.
EVENT
| Type of Event | Recall |
|---|---|
| Recall Number | Z-1820-2024 |
| Risk Class | Class 2 |
| Recall Qty | 183,248 |
| Classification Date | 2024-05-14 |
| Recall Link | Recall Link |
| Notes/Alerts | N/A |
| Recall Reason | A manufacturing issue could prevent the device from delivering instructional voice prompts to the user during use of the device. Visual instructional icons will still be present, but this issue could potentially lead to no therapy or a delay in therapy. |
DEVICE
| Manufacturer | Stryker/HeartSine Technologies Ltd |
|---|---|
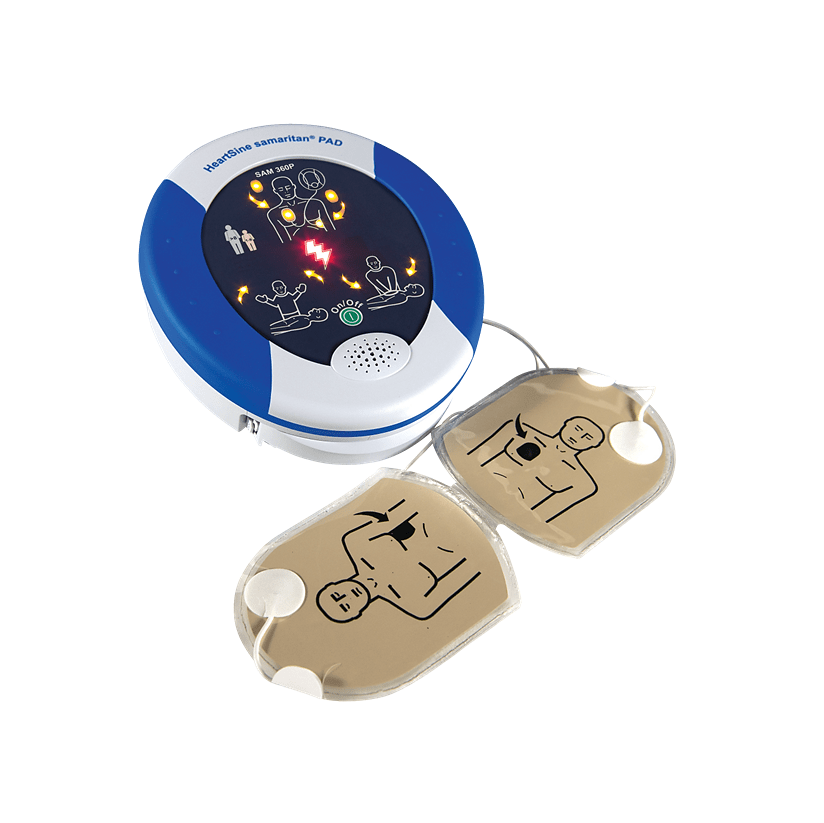
| Model Name | HeartSine Samaritan PAD |
| Model Number | SAM 350P, SAM 360P, SAM 450P |
| Part/Item/Catalog/ REF/Product Number | - |
| Device Description | Automated external defibrillators (non-wearable) |
| Serial Number(s) | Devices affected by this notification begin with the following prefixes and device codes: 16D, 16E, 16G, 17D, 17E, 17G, 18D, 18E, 18G, 19D, 19E, 19G, 20D, 20E, 20G, 21D, 21E, 21G, 22D, 22E, 22G, 23D, 23E, 23G, 24D, 24E, 24G Serial Numbers: The prefix (device identifier) consists of the manufacturing date (YY) and the device model (B, D, E, G, or H) and an 8-digit serial number string. Example: 16D00001234. REFER TO THE RECALL FOR MORE DETAILED SN INFORMATION. |
| Lot #/Exp Date(s) | N/A |
CUSTOMER/PATIENT ACTIONS
On 04/08/24 Stryker sent correction notices to customers informing them of the following: 1) If affected devices are found, follow the instructions to power cycle your device: a) Check the expiration date on the rear of the Pad-Pak and if passed, do not use and replace the expired Pad-Pak. b) Place the affected device face up on a flat surface and slide the Pad-Pak into the affected device until you hear the “double click” to indicate that the tabs on the right and left sides of the Pad-Pak are fully engaged. c) Verify that the green Status indicator is blinking to indicate the initial self-test routine has been performed and the device is ready for use. d) Press the On/Off button to turn on. e) Listen for, but do not follow, the voice prompts to ensure you can hear the prompts. -If you do not hear a prompt, contact your Authorized Distributor or HeartSine Technologies Technical Support at: heartsinesupport@stryker.com -If you hear prompt “Adult patient” and/or “Call for medical assistance”, no further action is needed. f) Press the On/Off button to turn off. If you have not heard a warning message and the status indicator continues to flash green, the device is ready for use. g) Carry out check once every three months and maintain awareness of this communication. h) Although this audio issue will not cause a warning message, if any other warning messages are played, or you see a red flashing status indicator, please refer to User Manual (General Troubleshooting). 2) Complete and return the response form to RSRecall@stryker.com. 3) If further distributed, please send an email to RSRecall@stryker.com. Stryker will work with you to ensure recipients are notified. 4) If you have any questions or concerns, please contact HeartSine Customer Support at +44 28 9093 9400, 9:00 A.M. to 5:00 P.M. (GMT), Monday Friday or by email at RSRecall@stryker.com. |
CONTACT INFORMATION
Report any adverse health consequences experienced with the use of this product to HeartSine Technologies Ltd. Events may also be reported to the FDA’s MedWatch Adverse Event Reporting program via:
Web: MedWatch website at www.fda.gov/medwatch
Phone: 1-800-FDA 1088 (1-800-332-1088)
Mail: MedWatch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787