Equiptrack products and services are based only on information supplied to Equiptrack. Equiptrack does not have the complete history of every unique piece of equipment. Use the Equiptrack search as one important tool, along with an equipment inspection and functional check, to make a better decision about purchasing your next piece of used medical equipment.
WHAT IS THIS?
A Urgent Field Safety Notice is communication sent by a manufacturer to users or customers in relation to a corrective action taken by the manufacturer for technical or medical reasons to prevent or reduce the risk of a serious incident in relation to a device made available on the market.
EVENT
| Type of Event | Urgent Field Safety Notice |
|---|---|
| Notice Reference | 32095 |
| Notice Qty | 140 |
| Notice Date | September 2024 |
| Notice Link | Link |
| Notes/Details | None |
| Reason | Complete electrical safety testing was not conducted during manufacturing of certain Giraffe and Panda iRes Warmers. Because the electrical safety testing in manufacturing was incomplete for these devices, there’s the potential for leakage current to exceed IEC 60601 limits. In the unlikely scenario that this occurs, it could potentially lead to adverse impact to the user or patient. |
DEVICE
| Manufacturer | GE Healthcare |
|---|---|
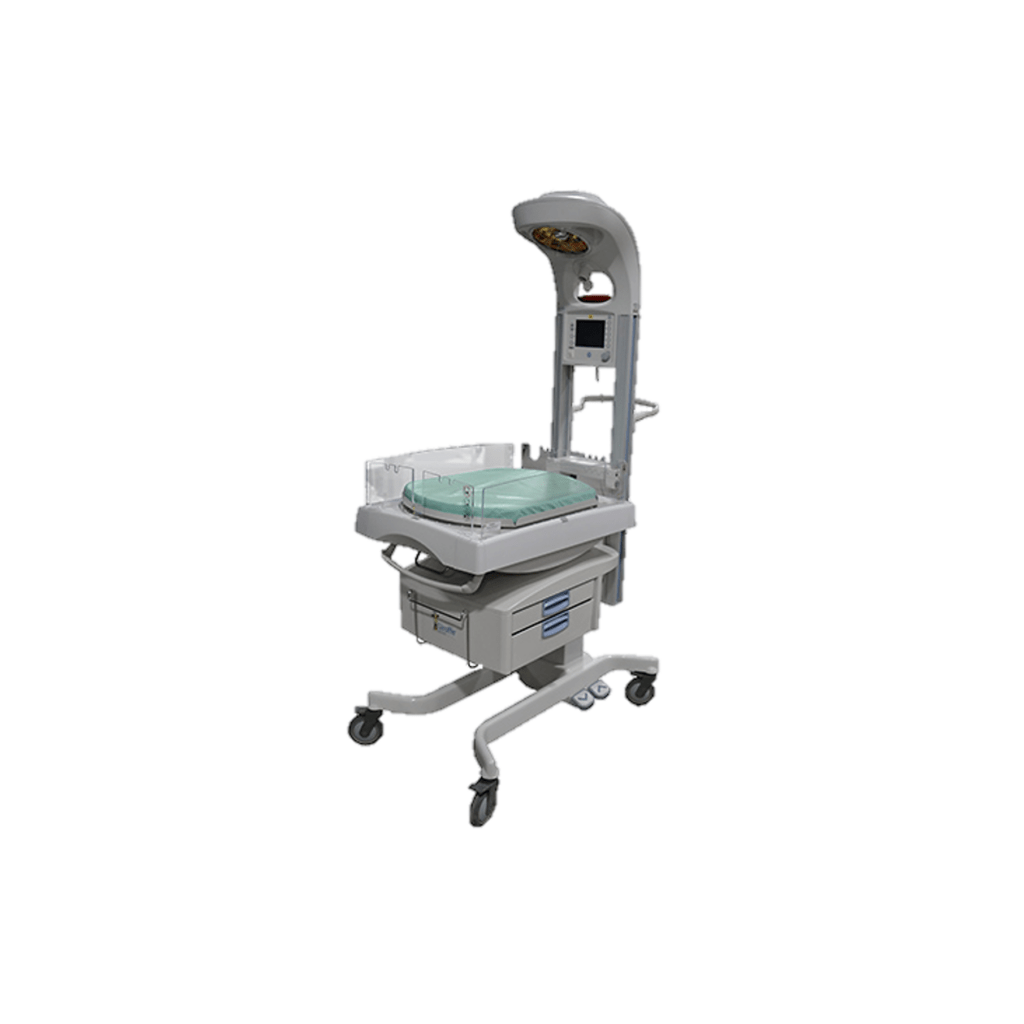
| Model Names | Giraffe and Panda iRes Warmers |
| Model Numbers | UDI: 840682103923 and 840682103893 |
| Part/Item/Catalog/ REF/Product Number | - |
| Device Description | Warmer |
| Serial Number(s) | Multiple. Follow Notice link for list. |
| Lot #/Exp Date(s) | N/A |
CUSTOMER/PATIENT ACTIONS
Prior to GE HealthCare inspecting and testing your systems, you can continue to use the affected Warmers by taking the following actions before each clinical use:
a. Inspect all power cords. Replace power cords with any damage.
b. Confirm proper grounding at your facility.
Please ensure all potential staff in your facility are made aware of this safety notification and the recommended actions.
Please retain this document for your records.
CONTACT INFORMATION
If you have any questions regarding this notification, please contact your GE Healthcare sales representative.
PLEASE EMAIL OR FAX THIS ACKNOWLEDGEMENT TO:
MIC.FMI32095@gehealthcare.com.