Equiptrack products and services are based only on information supplied to Equiptrack. Equiptrack does not have the complete history of every unique piece of equipment. Use the Equiptrack search as one important tool, along with an equipment inspection and functional check, to make a better decision about purchasing your next piece of used medical equipment.
WHAT IS THIS?
A Recall is a firm’s removal or correction of a marketed product that the FDA considers to be in violation of the laws it administers and against which the agency would initiate legal action, e.g., seizure. Recall does not include a market withdrawal or a stock recovery.
EVENT
| Type of Event | Recall |
|---|---|
| Recall Number | Z-0865-2024 |
| Risk Class | Class 2 |
| Recall Qty | 12,004 |
| Classification Date | 2024-01-30 |
| Recall Link | Recall Link |
| Notes/Alerts | N/A |
| Recall Reason | Issue can result in a double image artifact creating a ghost image with realistic features. |
DEVICE
| Manufacturer | GE Healthcare |
|---|---|
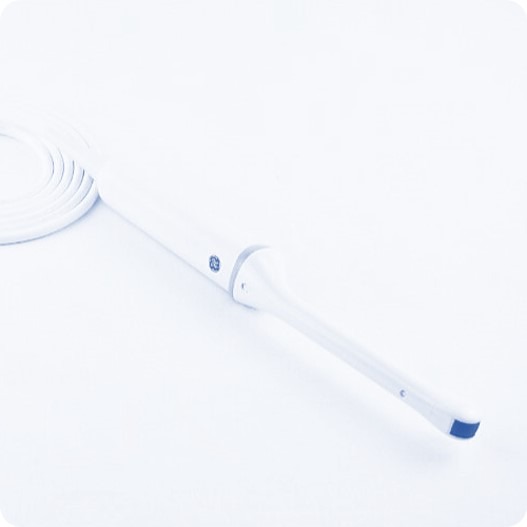
| Model Name | Voluson IC9-RS intracavitary probe |
| Model Number | J48691PJ |
| Part/Item/Catalog/ REF/Product Number | - |
| Device Description | The GE IC9-RS is an endocavity probe. This probe is used in gynaecological and obstetric examinations as well as in urological examinations. |
| Serial Number(s) | 1222552WX0, 1263125WX5, 1066771WX5, ... |
| Lot #/Exp Date(s) | N/A |
CUSTOMER/PATIENT ACTIONS
1. Ensure all potential users in your facility are made aware of this correction notification and the recommended actions.
2. You can continue to use your ultrasound system with all other probes.
3. Upon receipt of the letter, verify if your IC9-RS probe is functioning correctly prior to use by performing the Double Image artifact test provided in the letter. (Testing must be repeated monthly)
4. If a double image artifact is seen, do not use the probe and contact a GE HealthCare representative to get a replacement probe.
5. Complete and return the attached acknowledgement form to Recall.79072@ge.com. Please retain this document for your records.
CONTACT INFORMATION
Report any adverse health consequences experienced with the use of this product to BD. Events may also be reported to the FDA’s MedWatch Adverse Event Reporting program via:
Web: MedWatch website at www.fda.gov/medwatch
Phone: 1-800-FDA 1088 (1-800-332-1088)
Mail: MedWatch, HF-2, FDA, 5600 Fishers Lane, Rockville, MD 20852-9787